Nowadays, whenever someone mentions the word “antibody”, it will definitely catch your attention. People usually think about the human immune system and its role in the ongoing COVID-19 pandemic, and how our body can resist disease. The immune system is extremely complex, but almost everyone knows that antibodies are part of it, and they are vital to the body’s ability to recognize and neutralize invaders such as bacteria and viruses.
However, as important as antibodies for long-term immunity and disease avoidance, this is far from all their benefits. The incredible specificity of antibodies to their target antigens makes them powerful tools for biological research and clinical diagnosis such as rapid COVID-19 testing. The specificity of antibodies has also opened up a treatment that once belonged to science fiction, in which customized antibodies are like missiles, not only directly attacking specific proteins in the body, but sometimes even attacking specific parts of the body. protein.
However, to make these therapies work requires special antibodies: monoclonal antibodies.These are very Recently in the newsNot only can it be used as a possible treatment for COVID-19, it can also treat all diseases from rheumatoid arthritis to the most severe form of cancer. But what are monoclonal antibodies, how are they made, and how do they work?
Poly vs. Mono
Before delving into the details of monoclonal antibodies, it helps to have a general understanding of how the immune system works. Fortunately, the basics are very simple. In short, there are two immune systems in the human body: innate immunity and adaptive immunity. Both are composed of specialized white blood cells called lymphocytes. The innate immune system is a “quick attack” system that can quickly distinguish between friend and foe and digest invaders. The remnants of these invaders, mainly their protein fragments, are called antigens, which are presented to the lymphocytes of the adaptive immune system, which are covered with antibodies called immunoglobulins. These antibodies are a highly diverse group of proteins that represent the chemical memory of antigens that the body has encountered before. Any antigen that matches the antibody will bind to it, triggering a series of events, leading to a large accumulation of lymphocytes carrying the antibody, and then searching for and destroying the invader with great specificity.
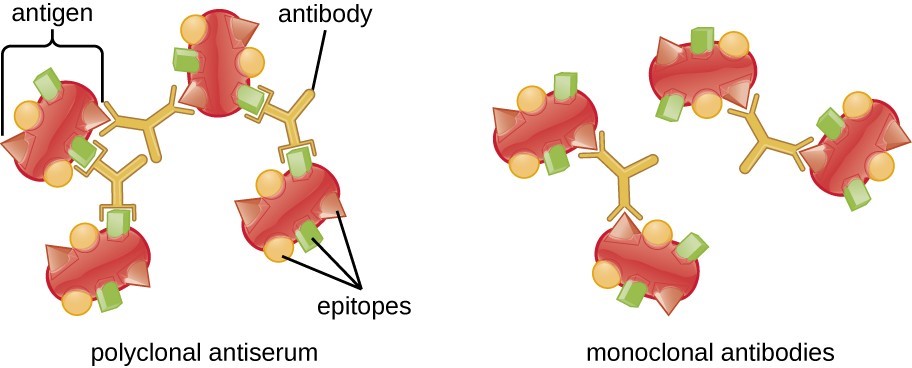
Although the antibodies produced by the adaptive immune system are highly specific to specific antigens, they still have some “room for maneuver”. The antigen presented to the adaptive immune system is actually very large, and it is entirely possible that there are antibodies against different features (called epitopes) of the same protein. This is an advantage because it increases the likelihood that the adaptive immune system will be able to recognize the antigen, no matter how it is chopped up by the innate immune system. This is also helpful in case the virus mutates, which may change the epitope enough to no longer be recognized. Antibodies against multiple epitopes are called polyclonal antibodies, which provide redundancy and help increase the chance of a strong immune response.
Although I have described how the immune system works in your body so far, it is important to remember that antibodies are also powerful tools for research and clinical diagnosis. The ability to create populations of antibodies that can bind to specific proteins is a great boon for biological research. Antibody production usually involves injecting a target antigen into a rat or mouse, collecting leukocytes from the animal, and then purifying the antibody to obtain an antibody that binds to the target antigen. This is a process that takes a lot of time—three months or more is not uncommon—and a lot of professional skills. The end result is a set of polyclonal antibodies.
Although traditional antibody production technologies have done a lot of heavy work in biomedical research for many years, sometimes the polyclonal antibodies they produce are not tools for this work. In some cases, more binding specificity is required, and this is the purpose of monoclonal antibodies. Monoclonal antibodies, usually abbreviated as “mAbs”, are antibodies that recognize a single epitope of the target antigen. This can be a very powerful research tool because antibodies that bind only to a single region of a large protein can be created. This can be used to explore the function of the region; for example, a monoclonal antibody that only binds to a specific epitope can be used to detect whether the region is a binding site for another protein by physically blocking access to it.
Cancer cells are doing well
Although the natural immune system is basically a polyclonal antibody factory, this does not mean that monoclonal antibodies will not appear naturally. Unfortunately, they are most common in patients with multiple myeloma, a blood cancer that affects immune-related blood cells. All cancers are characterized by overproduction of specific tissue types, and natural myeloma cells provide researchers with a tool to create large numbers of identical cells—clones—each of which produces an antibody.
However, the trick is to figure out how to program myeloma cells to produce specific antibodies, and when all of these were first explored in the 1970s, molecular biology was still in its infancy. Therefore, the researchers had to modify it slightly to achieve this. The basic idea they put forward is to fuse immortal myeloma cells with cells that carry antibodies against the target antigen. These hybridoma cells each carry a gene for a single antibody, and once the work to create them is completed, they can grow in large numbers.
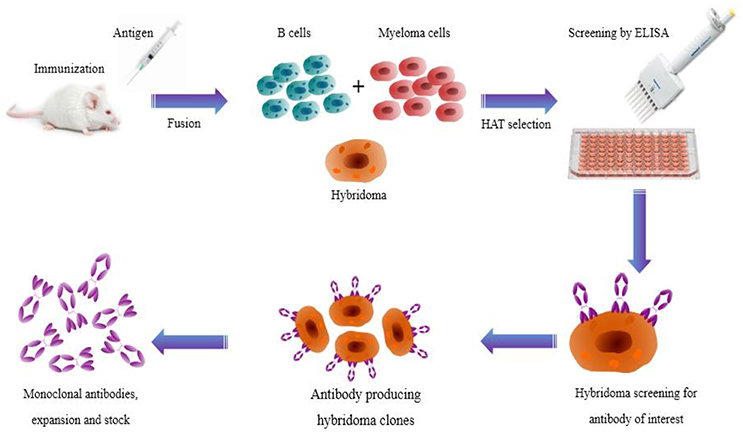
Hybridoma cells are difficult to produce. The first is the problem of cultivating large numbers of antibody-producing cells (called B cells) in mice or other mammals. The B cells must then be fused with myeloma cells, either by chemical treatment by treating the cells with polyethylene glycol, or by using an electric field. Another problem is to separate the few cells that successfully fused together from the unfused myeloma and B cells. This is achieved by using myeloma cells that are sensitive to the chemotherapy drug aminopterin. The successfully fused myeloma cells will be “rescued” by the full version of the genes in the B cells and will be able to survive in the growth medium containing the drug.
The next trick is to transform the polyclonal population of hybridoma cells into multiple monoclonal populations. This is done by diluting the surviving fused cells to an average of only one cell in a given volume of growth medium. Putting this volume into each well of a microtiter plate allows a single cell to grow, producing a clonal cell line that carries genes to produce antibodies against a single epitope. Then, finding a cell line that reacts with a specific epitope is a matter of screening all hybridoma lines using various immunochemical methods, such as fixing the antigen on a solid substrate, and then only binding to hybridomas specific to it.
In the decades since hybridoma technology was first explored, many other mAb production methods have been developed. Phage display, in which viruses that infect bacteria are modified to express antibodies on their protein shells, can be used to screen a large number of antibodies against specific antigens, and then the DNA encoding the antibodies can be easily cloned. Transgenic mice with human antibody genes can also be used to produce mAb libraries; almost all currently approved monoclonal antibody therapies on the market are developed through genetically modified mice.
Medical guided missile
From the above example of using mAbs to detect protein binding domains, this is a very short leap. It can be seen that they can be used not only for diagnosis, but also for therapy. The specificity of monoclonal antibodies makes targeted therapy very targeted, rather than oral drugs and hope that it will be transported throughout the body to the therapeutic target. This is where the monoclonal antibody has a reputation as a medical “missile”.
However, when you consider how mAb therapy works, the missile metaphor begins to become a bit out of date. In cases where missiles usually need to fire warheads to function, in mAb therapy, sometimes the fact that they can bind to specific proteins is enough to be a weapon. This is especially true for the treatment of autoimmune diseases such as Crohn’s disease and rheumatoid arthritis, where the patient’s own immune system mistakes its own cells for invaders and starts attacking them.
These diseases are treated with monoclonal antibodies that target and bind tumor necrosis factor-α (TNF-α), a protein that regulates the immune system and can inhibit the self-destructive cascade. Some cancer therapies use a similar approach, combining mAbs with certain proteins that regulate cell division, thereby targeting them for elimination by the patient’s immune system.
However, some mAb therapies also carry payloads to their targets. In some cases, radioisotopes can be attached to mAbs to directly provide a certain dose of radiation to cancer cells; other mAbs can provide drug molecules or prodrug molecules with molecular precision, which can then be converted to their active forms.
COVID monoclonal antibody
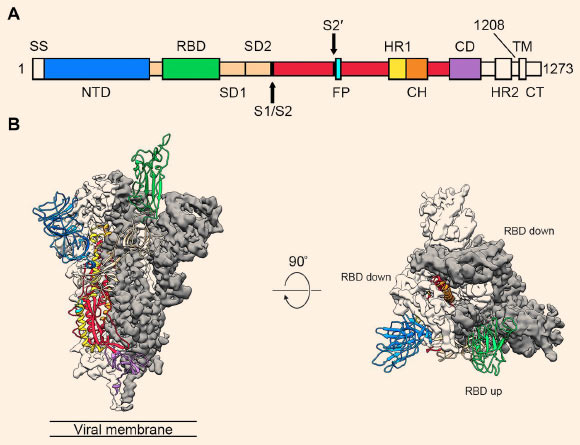
All of this has given us an understanding of the current pandemic and how to use monoclonal immunotherapy to help COVID-19 patients. To date, the US Food and Drug Administration has approved three mAb immunotherapies for COVID-19 under emergency use authorization (EUA). Two of them are currently recommended and used for hospitalized patients with mild to moderate COVID-19. One is a mixture of two monoclonal antibodies, called Cassirezumab and imdevimab-you can determine whether a drug is a monoclonal antibody in the following ways-Monoclonal antibodies Suffix-This is interesting because although each mAb binds to the legendary spike protein of the SARS-CoV-2 virus, each of them binds to a different but overlapping epitope of the protein.
Both epitopes are in the receptor binding domain (RBD) of the spike protein, which means that once the mAb binds to the spike protein on the circulating virus particle, it can no longer bind to its receptor, and ACE-2 is The body is located in the epithelium of the respiratory tract. The result is that the virus can no longer enter the cell and replicate, which is expected to reduce the viral load and speed up recovery.
For casirivimab/imdevimab sold by Regeneron under the name REGEN-COV, The numbers look good — Administration of mAb therapy (intravenous infusion or a set of four subcutaneous injections) within the first 10 days of symptoms can reduce the risk of mild to moderate COVID progression by approximately 70%. It also has indications for preventive use after exposure to immunocompromised individuals.
Monoclonal antibodies have changed the rules of the game in biological research and treatment of various terrible diseases. The development of new technologies makes them easier to manufacture, and their use as medical missiles should be expanded.