When you suffer from diabetes in your life, there will be moments in any day without any thoughts about insulin. Insulin is actually my first coherent thought every morning, when I check my daughter’s blood sugar level while she is still asleep, and the last thought when I turn off the lights, to make sure she has enough insulin in her insulin pump. Come through the night. In between, due to the constant need to calculate doses, adjust levels, add corrections to unexpected snacks, or just check the refrigerator and count the number of spare vials we have on hand, insulin is a common, even regular My thoughts on unwelcome intruders.
Now, as my daughter grows up and seeks to become more independent like any teenager, new ideas about insulin are beginning to emerge. Insulin is expensive, Although we have good insurance, this situation will always change instantly. But even so, insulin must flow-she has no choice in this matter. Therefore, I think that in the context of the growing bio-hacking movement, it will be instructive to study how to manufacture insulin on a commercial scale. They hope to establish a more distributed insulin production system. Their goal is to make insulin affordable, and out of a vested interest, I want to know if they have a chance to achieve this goal.
This is a lot of pigs
To understand what is involved in making artificial insulin, the best starting point is to understand natural insulin. Insulin is a hormone involved in regulating blood sugar levels and is secreted by the pancreas. Special cells called beta cells sense the glucose level in the blood (usually soaring after a meal) and secrete insulin into the blood as a response. Insulin spreads around the body quickly, and interacts with cells by stimulating the glucose transport system of the cells to absorb blood sugar to meet their metabolic needs.
However, in type 1 diabetes, the ability of the pancreas to produce insulin has been destroyed. The reason for this is not clear, but it is generally believed to be at least partly related to autoimmunity, that is, a person’s own immune system recognizes pancreatic beta cells as foreign cells and destroys them. As a result, people with type 1 diabetes partially or completely lack the ability to sense and respond to elevated blood sugar, which means that they must take regular insulin injections to survive. Type 2 diabetes is a completely different disease, partly because the body is not sensitive to insulin. Although some people with type 2 diabetes also take insulin, it can be treated with a variety of other drugs.
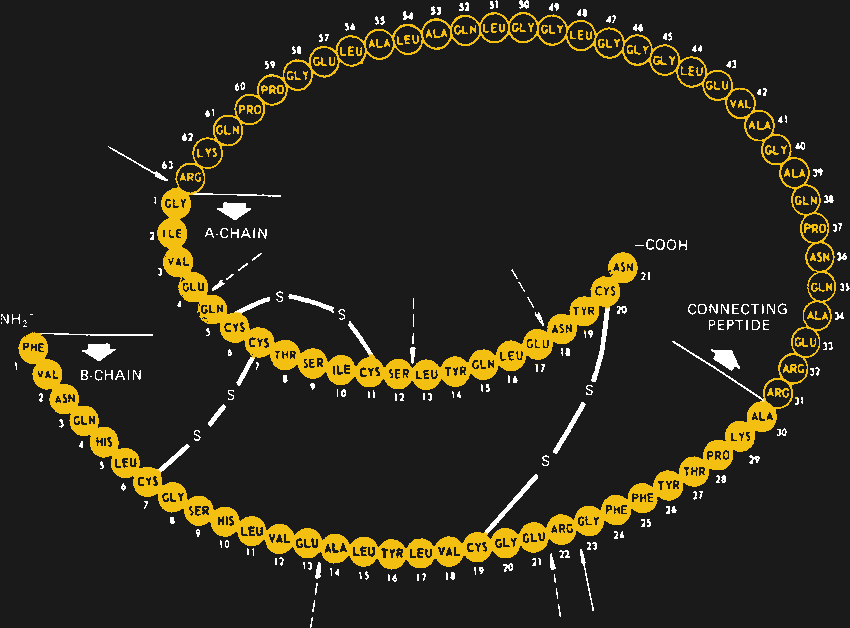
When Canadian doctors Banting and Best first identified it as a diabetes drug in 1920, it was surprising to learn that insulin was actually a protein. Before that, people had always assumed that all hormones were small molecules, so it was discovered that insulin is actually a protein consisting of 51 amino acids in two independent chains, which are connected together at three positions by disulfide bonds. The discovery was shocking. It will also open the door to recombinant insulins currently on the market today.

However, in the first 60 years or so of its commercialization, insulin was strictly derived from animal sources, mainly from the pancreas of pigs. The amino acid sequence of insulin is highly conserved among species, and even insulin from fish has clinical effects on humans. The difference between porcine insulin and human insulin is only one amino acid, but its purification is a difficult process. The internal organs of the two-ton slaughterhouse produce only 250 milliliters of insulin. Through a thoroughly disgusting process of grinding, shredding and extracting insulin, it is purified to a level that is free of contaminants and does not cause an immune response when injected. However, sometimes it still does.
In the 1980s, with the introduction of recombinant insulin, all of this changed. Recombinant insulin was made from genetically engineered microorganisms. By putting the human insulin gene into a small circle of DNA called a plasmid, and then inducing these plasmids into bacteria or yeast, a cell line that can produce large amounts of insulin in a way that is easy to amplify and amplify is created. Much easier than purification from mammalian tissues. It also has the same advantages as natural human insulin, up to the last amino acid. Moreover, it opens the door to genetic engineering to adjust the amino acid sequence to produce different types of insulin with specific characteristics.
expand
Today, most insulin is produced in common bacteria Escherichia coli, Although some manufacturers prefer to use Saccharomyces cerevisiae in their processes. No matter what kind of microorganism, the process of producing a batch of insulin is very simple. Transgenic cells are used to inoculate large volumes of liquid culture medium in devices called bioreactors. This is basically a huge stainless steel tank that holds the fermentation culture, as well as heaters, agitators, aerators and a series of sensors for controlling and monitoring the growth process. There are usually multiple bioreactors connected in series, and the output of the smaller one is sent to the larger one to gradually increase the amount of culture.
The transgenic microorganisms are allowed to grow until they reach the maximum growth rate, at which time they are harvested from the bioreactor. The first step is filtration, which separates the insulin-filled cells from the spent growth medium. Then a high-pressure homogenizer is used to split the cells, leaving a mixture of cell debris and insulin inclusion bodies, which are a bit like hormone crystals formed in the cells during the fermentation process.
This is where things become sensitive. So far, insulin is very safe inside the cell. However, once the cells split, all of their proteins, including enzymes (proteases) that degrade and recycle other proteins, begin to act on insulin, degrading it and reducing production. Avoiding this situation requires choosing the right buffer, adding the right protease inhibitor, and keeping everything at a low temperature. It is also important to act quickly-the longer insulin is exposed to cell debris, the greater the chance of degradation.
After a series of lysis steps to further separate insulin from cell debris, a round of post-processing may be required. In the body, insulin is expressed as a single long-chain amino acid called proinsulin, which folds back to itself and forms a tertiary structure between distant cysteine residues. Once this structure is established, the loop is cut in two places, leaving behind the active double-stranded hormone. Artificial insulin needs to replicate this structure; depending on the manufacturer, this can be achieved by growing the A and B chains separately and combining them chemically, or by producing proinsulin and processing it afterwards.
Either way, the almost finished insulin still needs some final purification. This is usually done using affinity chromatography, where the insulin produced by microorganisms has a protein “tail”. The tail allows the protein to bind to the antibody, and the antibody is attached to a solid matrix. When the solution containing the fusion protein flows through the matrix, anything that does not have a protein tail will flow out with the waste, causing the insulin fusion protein to stick to the matrix. Then the pure fusion product is released from the column by adjusting the pH value of the solution, cleaved from the protein tail, and final purification is performed using reversed-phase chromatography before passing strict QC inspection and final packaging.
The smaller the better
Although commercial insulin production is of course complicated and expensive, this is not because the process itself is difficult. Creating and maintaining genetically modified cell lines, expressing fusion proteins, and purifying final products are things that graduate students in biological laboratories around the world do every day. The complexity of commercial insulin production comes from the fact that it must be expanded to an economically feasible level, resulting in huge laboratories with rows of bioreactors, endless stainless steel pipes, and extremely complex command and control systems to ensure that it all works. It is appropriate to compare modern biologics factories that produce not only insulin but also other biological drugs such as immunotherapy drugs with semiconductor factories: both capture the essence of the problem and the potential for scale-and manufacture in your garage Semiconductors are entirely possible, and doing so will never be commercially viable.
Accepting this cruel reality is the key to solving the problem of insulin supply, at least in their opinion Open Insulin Foundation. OIF is a biohacking team with an extensive geographic footprint. It is a non-profit organization focused on creating a community-scale system that can produce safe and affordable insulin. In the past six years, their volunteers have been busy constructing genetically modified bacteria, studying the cultivation process and equipment, and dealing with cumbersome purification steps. They have successfully seen the process from the entire process to the finished product.
Now that OIF has figured out the science, the next step is to expand it appropriately.exist 2020 presentationLouise Lassalle, the group’s communications manager, detailed the equipment needed to produce insulin on a medium scale—larger than batches produced on a desktop computer, but far less than the capacity of commercial biologics factories. It’s not cheap—equipment worth about $1 million. However, this investment will produce enough insulin for 14,000 diabetic patients, which means that it is conceivable that a well-functioning community-level insulin factory can reach about $70 per person. Taking into account all factors from raw materials to wages, rent and utilities, the factory will produce insulin at a price of about $6 per bottle.
To be sure, there are huge legal and regulatory hurdles that may be insurmountable. It is likely that the insulin industry has a vested interest in keeping prices high—at least in the United States—will let their legal war dogs go, and close the group if they get close to achieving their goals. If they really want to use their insulin for humans, of course they must deal with the Food and Drug Administration. On the other hand, in the long run, the efforts of the Open Insulin Foundation and other biohackers working to replace sources of insulin may win, because it shows that making insulin is not as difficult as we might think, and even if it is advantageous A viable business, it is also possible to expand everything to a large scale is not always in the best interests of consumers.
This leads to another point that may be more important. If the past year and a half has not taught us anything else, it is the need to look at long supply chains with suspicion. When a pharmaceutical company builds a large factory for financial reasons, it creates a single point of failure that may not need to exist. We have seen how easily the supply line can be squeezed, and it seems foolish to design a system that has the potential to shut down the supply of any drug in the world, especially for drugs as important as insulin. OIF’s vision of a distributed micro-factory for insulin production now seems more sensible, and as time goes by and complex systems become increasingly unreliable, it may only become a better idea.